17 The I1 values of Li, Be and C are 54 eV/atom, 932 eV/atom and 1126 eV/atom The I1 value of Boron is 1) 136 eV/atom 2) 9 eV/atom 3) 145 eV/atom 4) 215 eV/atom 18 The I1 of potassium is 4339 eV/atom, the I1 of sodium 1) 4339 2) 221 3) 5138 4) 1002 wwwsakshieducationcom wwwsakshieducationcom a) The first ionization energies (I1) of these elements are lower than the corresponding values for Group 2 elements However, the second (I2) and third (I3) ionization energies are much higher This is because the first electronThe heats of formation at 25° C, from amorphous boron and hydrogen, are 673 ± O52 kcal/ mole for diboranc (gas) >l nd 1299 ± O39 for pentaborane (gas) 1 Introduction In order Lo ascertain the bond energy relations in the boron hydrides, precise values of the heats of forma bon of these substances from the elements are necded
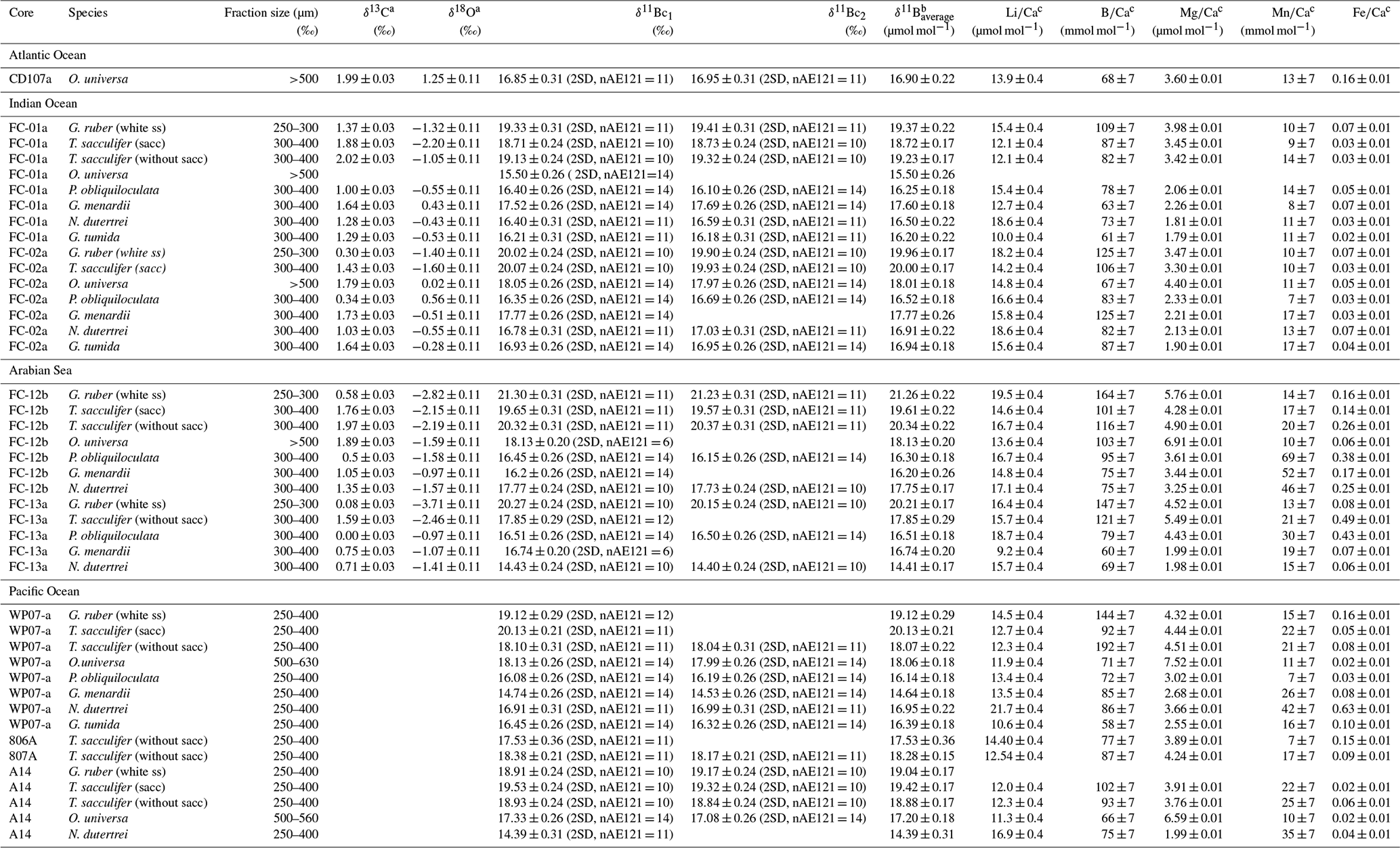
Bg Seawater Ph Reconstruction Using Boron Isotopes In Multiple Planktonic Foraminifera Species With Different Depth Habitats And Their Potential To Constrain Ph And Pco2 Gradients
Is boron valuable
Is boron valuable-Boron 11 Metal (Boron11) is a stable (nonradioactive) isotope of Boron It is both naturally occurring and a produced by fission Boron 11 Metal is one of over 250 stable Metallic isotopes produced by American Elements for biological and biomedical labeling, Peaches Peaches are high in boron, as well as vitamins C and A One medium peach contains 080 milligrams of boron and just 63 calories 9 Fresh, frozen or canned peaches are all good sources of boron Bite into a fresh, juicy peach as a snack, dice them up to make peach salsa, or toss some frozen peaches into a smoothie




Effects Of Boron Containing Compounds In The Fungal Kingdom Sciencedirect
Boron, #"B"#, is located in period 2, group 13 of the periodic table, and has an atomic number equal to #5# This means that a neutral boron atom will have a total of #5# electrons surrounding its nucleus Now, your tool of choice here will be boron's electron configuration, which looks like this #"B " 1s^2 2s^2 2p^1# Since you have five electrons, you willTable44B shows the individual comparison of treatment means The highest value of LAI (336) obtained at no stress level (I1) The minimum value of LAI (277) was obtained at drought 30 days after sowing (I2) but it was at par with LAI value (0651) drought stress at 60 days after sowing (I3) Appendix 45 shows data taken 85 days after sowing Boron may be natural and fully capable of helping you to manage your testosterone levels;
Some of these anomalous properties are discussed below Except for boron, the compounds of the elements of the boron family like tetrahedral M (OH) 4 – and octahedral M (H 2 O) 6 3 (where M denotes the member of boron family) exists in an aqueous medium The maximum covalence of boron is 4 due to the absence of d orbitalsBoron is a mineral that is found in food such as nuts and the environment People take boron supplements as medicine Boron is used for boron deficiency, menstrual cramps, and vaginal yeastBoron (B) is a chemical element with an atomic number 5 that belongs in the Period 2 and Group 13 in the periodic tableIt is a lowabundant metalloid that is a poor electrical conductor at room temperature Natural boron exists in 2 stable isotopes however pure boron is hard to prepare due to contamination by different elements It is also found for applications including ceramics, high
Hexagonal boron nitride is very similar to graphite The band structure of graphite has been investigated extensively, and a great deal of theoretical work (mostly recent) has also been done on hexagonaL boron nitride ' ' Still, the calculated theoretical values for the direct optical band gap Ee in hexagonal boron nitride are quite scatteredBoron Boron Compounds In its compounds boron shows an oxidation state of 3 The first three ionization energies of boron, however, are much too high to allow formation of compounds containing the ion; (Dibenzoylmethanato)boron difluoride (DBMBF2) and the three indenopyridine systems used were prepared according to the reported methods (I1 and I3) and I2 respectively Structural formulas of all the compounds used in this study




Boron Containing Minerals Of Commercial Importance Download Table



A Crossed Beam And Ab Initio Investigation Of The Reaction Of Boron Monoxide 11bo X2s With Acetylene C2h2 X1s G Physical Chemistry Chemical Physics Rsc Publishing
There are two groups of BNa distances, one with an average value of 2798 A;Uses Amorphous boron is used as a rocket fuel igniter and in pyrotechnic flares It gives the flares a distinctive green colour The most important compounds of boron are boric (or boracic) acid, borax (sodium borate) and boric oxide These can be found in eye drops, mild antiseptics, washing powders and tile glazesAt °C (68°F or K) at standard atmospheric pressureIn Imperial or US customary measurement system, the density is equal to pound per cubic foot lb/ft³, or 1353 ounce per cubic inch oz/inch³



Repository Tudelft Nl Islandora Object Uuid 904db2dd B63a 4d17 8742 1ebbf1627e01 Datastream Obj Download



Comparative Study Of Gas Dynamic Processes In Inductively Coupled Argon Hydrogen Plasma Containing Boron Trichloride And Boron Trifluoride Springerlink
The symptoms of too much boron include nausea, vomiting, diarrhea, rashes, headaches, and convulsions Very high amounts of boron can cause death The daily upper limits for boron boron include intakes from all sources—food, beverages, and supplements—and are listed below in milligrams (mg)Notes on the properties of Boron Specific Heat Value given for solid rhombic form Up to date, curated data provided by Mathematica's ElementData function from Wolfram Research, Inc Click here to buy a book, photographic periodic table poster, card deck, or 3D print based on the images you see here! Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structureThe chemical symbol for Boron is B Significant concentrations of boron occur on the Earth in compounds known as the borate minerals There are over 100 different borate minerals, but the most common are borax, kernite, ulexite etc Natural boron
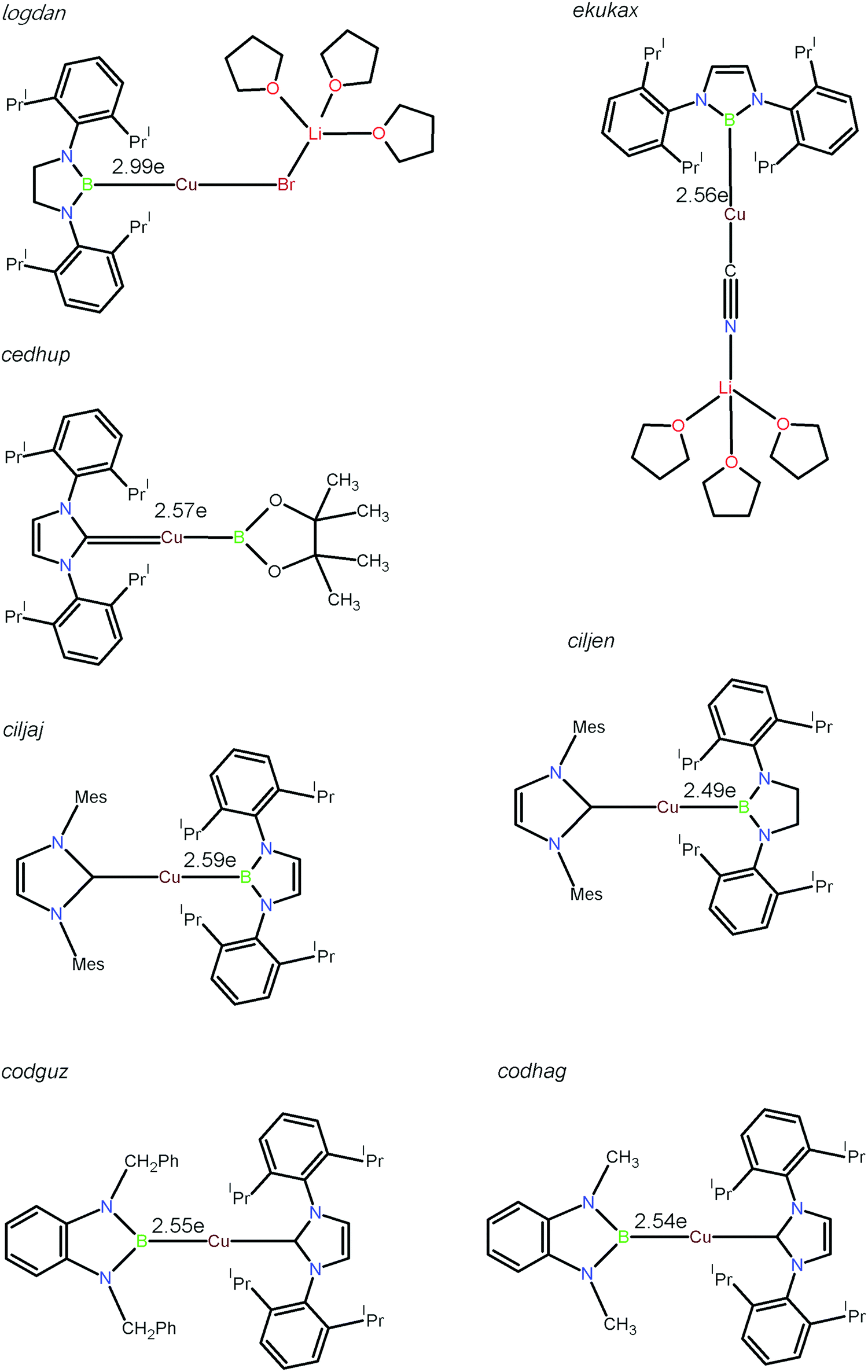



On The Nature Of The Boron Copper Interaction Topological Study Of The Electron Localisation Function Elf New Journal Of Chemistry Rsc Publishing Doi 10 1039 C8njd
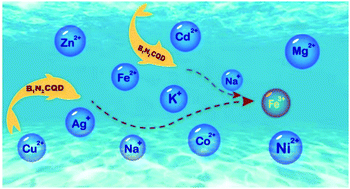



Highly Fluorescent Nitrogen And Boron Doped Carbon Quantum Dots For Selective And Sensitive Detection Of Fe3 Journal Of Materials Chemistry B Rsc Publishing
Boron has two naturally occurring and stable isotopes, 11 B (801%) and 10 B (199%) The mass difference results in a wide range of δ 11 B values, which are defined as a fractional difference between the 11 B and 10 B and traditionally expressed in parts perThe alkali metal of any given period has the _____ I1 value in the period, which reflects the relative ease with which its outer _____ electron can be removed As a result, the alkali metals are all very _____, readily losing one electron to form ions carrying a 1 charge Going from boron to carbon, the nuclear charge is _____ due to theHowever, on its own it is limited For starters, you can only use it for 2 weeks at a time, and two, abuse it and it can trigger excess estrogen, toxicity, and other side effects



1
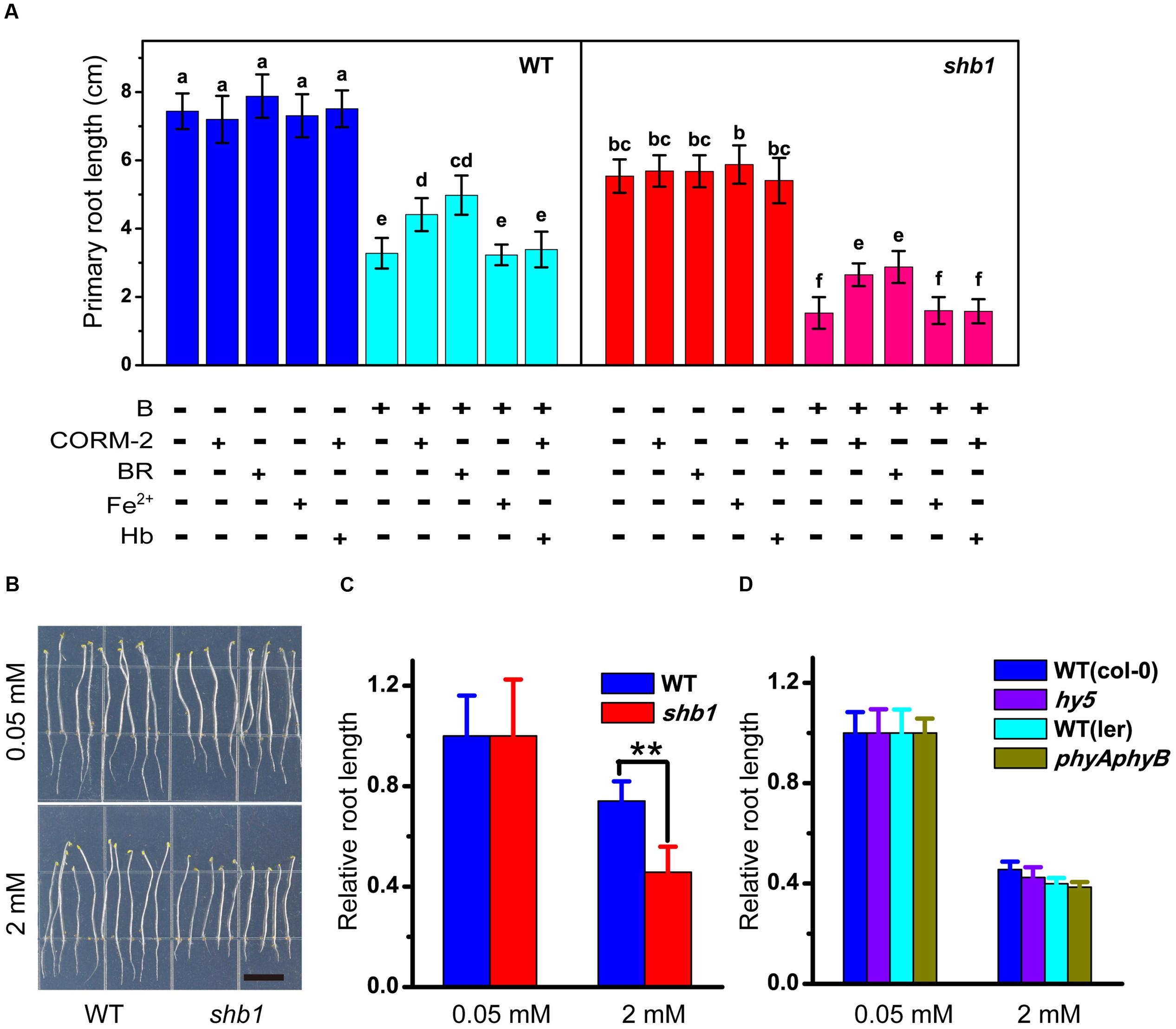



Frontiers Shb1 Hy1 Alleviates Excess Boron Stress By Increasing Bor4 Expression Level And Maintaining Boron Homeostasis In Arabidopsis Roots Plant Science
The other with a mean value of 2551 A The presence of the somewhat short BNa distances, suggests that sodium bonding to boron is important to the stabilization of the structure The measured d,, T and N values are presented in Tab 1 3b Me a sure me nts of the C I1 Spectral Line The values of d, and w, for 678 nm C I1 spectral line from multiplet No 14 have not been measured before CO, has been used as a working gas at a filling pressure of 130 Pa A condenser battery of 8 pF capacity was charged to 64 J of energyThe Easiest Way to Get Enough Boron While many foods contain boron, getting your 3 mg of boron each day through diet alone could be challengingMost fresh fruits, vegetables, and even honey contain between 01–05 mg boron Animalbased foods like chicken, milk, and tuna provide just 001–006 mg, so fruit and plantbased sources are your best bet



2




Effects Of Boron Containing Compounds In The Fungal Kingdom Sciencedirect
Boron weighs 234 gram per cubic centimeter or 2 340 kilogram per cubic meter, ie density of boron is equal to 2 340 kg/m³;A value of 15 x cm2/sec was obtained for the diffusion coefficient boron electrodes in a 02 WaNO solution varying in pH from 3 Tafel slopes are swnmarized in Table I1 for the electrolyte studies D MMEtD POTENTIALSThe I1 values of Li, Be and C are 54 eV/atom, 932 eV/atom and 1126 eV/atom The I1 value of Boron is 1) 136 eV/atom 2) 9 eV/atom 3) 145 eV/atom 4) 215 eV/atom 18




Crystal Structure Of Boron Rich Metal Borides Wikiwand




Corrosion Current Value In 3 Nacl For Coatings Obtained At Various Download Scientific Diagram
Boron is produced domestically only in the State of California Boron products sold on the market are produced from a surface mine, underground mines, and in situ and from brine The United States and Turkey are the world's largest producers of boron Boron is priced and sold on the boron oxide basis, which varies by ore and compound and on the absence or presence of sodium What is Boron Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure The chemical symbol for Boron is B Significant concentrations of boron occur on the Earth in compounds known as the borate minerals There are over 100 different borate minerals, but the most common are borax, kernite, ulexite etc Natural boronThe electronic configuration of Boron (At No 5) is 1s^2, 2s^2, 2p^1 So, there are only two shells The second shell contains one 2s orbitals and three 2p orbitals resulting total four orbitals in second shell The total number of orbitals availa




Atomic Arrangement And Mechanical Properties Of Chemical Vapor Deposited Amorphous Boron Sciencedirect




Finctor Bnct Fund Sp
Boron can have a slight impact on your testosterone levels, and you may very well notice some differences But it's less likely that you'll see any changes in symptoms of erectile dysfunction (ED)Boron11 is the stable isotope of boron with relative atomic mass , 801 atom percent natural abundance and nuclear spin 3/2 A trace element with the atomic symbol B, atomic number 5, and atomic weight ; There is overwhelming data for the safety of boron Boric acid has a LD 50 of 2660 mg/kg (rat, oral) which is almost as high as regular table salt at 3000 mg/kg (rat, oral) Boron is found in fruits, vegetables and it is considered an essential plant nutrient Boric acid is used in eye wash solution and many creams as well



A Crossed Beam And Ab Initio Investigation Of The Reaction Of Boron Monoxide 11bo X2s With Acetylene C2h2 X1s G Physical Chemistry Chemical Physics Rsc Publishing




Materials Free Full Text Influence Of Amorphous Boron Grain Size High Isostatic Pressure Annealing Temperature And Filling Density Of Unreacted Material On Structure Critical Parameters N Value And Engineering Critical Current Density
The s and pblock elements show a larger range of I1 values than do the transition metal elements Generally, the ionization energies of the transition metals increase slowly from left to right in a period The fblock metals also show only a small variation in the values of I1Click here👆to get an answer to your question ️ The IE1 values of Li, Be and C are 54 eV/atom, 932 eV/atom and 1126 eV/atom The IE1 value of boron is Join / LoginThus, in all its compounds boron is covalently bonded That is, one of boron's 2s electrons is promoted to a 2p orbital, giving the outer electron configuration 2s12p2;




21 The Ionization Potentials Of Li And K Are 5 4 And 4 3 Ev Respe




Boron Retention And Percentage Boron Released From The Wood Blocks Download Table
Layers given in Table I1 are average values of filar micrometer measurements of the sections shown in Figure 2 It may be seen from these sections, particularly Figure 2(c), that the thickness of the boron and polyimide layers is quite uniform Some breaksFig 1 Results of hardness values As shown in Fig 1, without boron samples after heat treatment were be 343 to 345 HB In particular, hardness values of the sample 30ppm boron addition were highest values obtained However, the boron addition over 30 ppm showed to decrease in hardness valuesBoron helps keep your teeth and gums healthy through reducing inflammation and improving bone and tissue repair There's an interesting study that came out in 13 that found boron would helps the tooth building cells in such a way it's believed that boron could be used in bone and tooth tissue engineering This is a long way off from



Royalsocietypublishing Org Doi Pdf 10 1098 Rspa 1954 0162
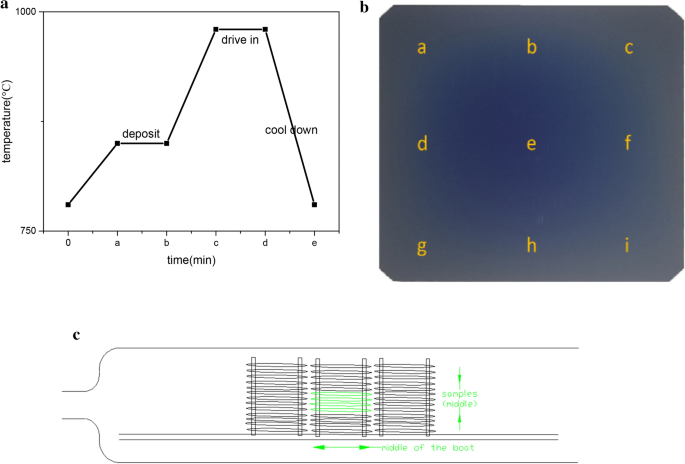



Study Of Boron Diffusion For P Emitter Of Large Area N Type Topcon Silicon Solar Cells Springerlink
2 liquid borane/air propellant i1 3 approximate flame compositions, temperatures and jet conditions 13 4 values of ocrit for y = 14 16 5 distributions of bcontaining products after nozzle expansion frozen and with and without condensation 30 4Quantity Atomic Unit Value in SI Energy ħ2/m e a 0 (Hartree) 436 x 1018 J Charge e 160 x 1019 C Length a 0 529 x 1011 m Mass m e 911 x 1031 kg In SI units In atomic units CHEM6085 Density Functional TheoryBoron is a metalloid with an atomic number of 5 in the periodic table of elements It's found in an amount of percent in the Earth's crust Being a member of the boron family of periodic table elements, this chemical substance has three valence electrons While boron is labeled as a nonmetalic element, the other chemical elements
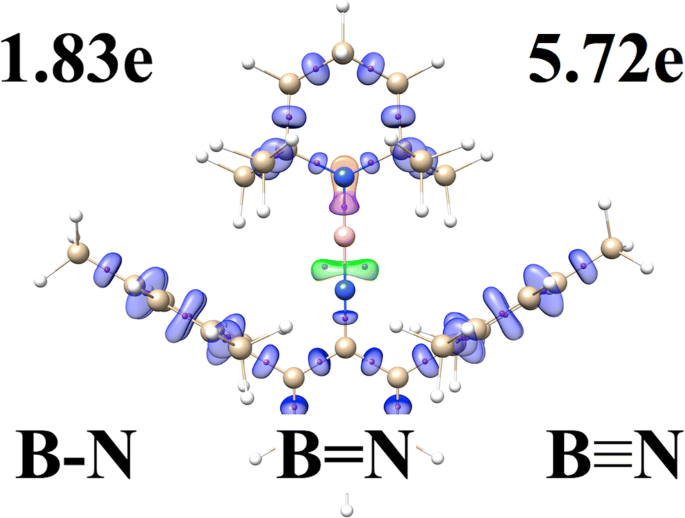



The Nature Of Multiple Boron Nitrogen Bonds Studied Using Electron Localization Function Elf Electron Density Aim And Natural Bond Orbital Nbo Methods Springerlink




Boron 3mg Vitalchoices Aruba
Boron has been useful to mankind in the form of different compounds The existence of this element was first discovered by Jöns Jakob Berzelius It is known to have wide variety of uses from medical to industrial purposes Read this ScienceStruck article to know more about what the element boron is used for



Www Degruyter Com Document Doi 10 1515 009 Pdf




Boron Content Of Plant Samples Download Table
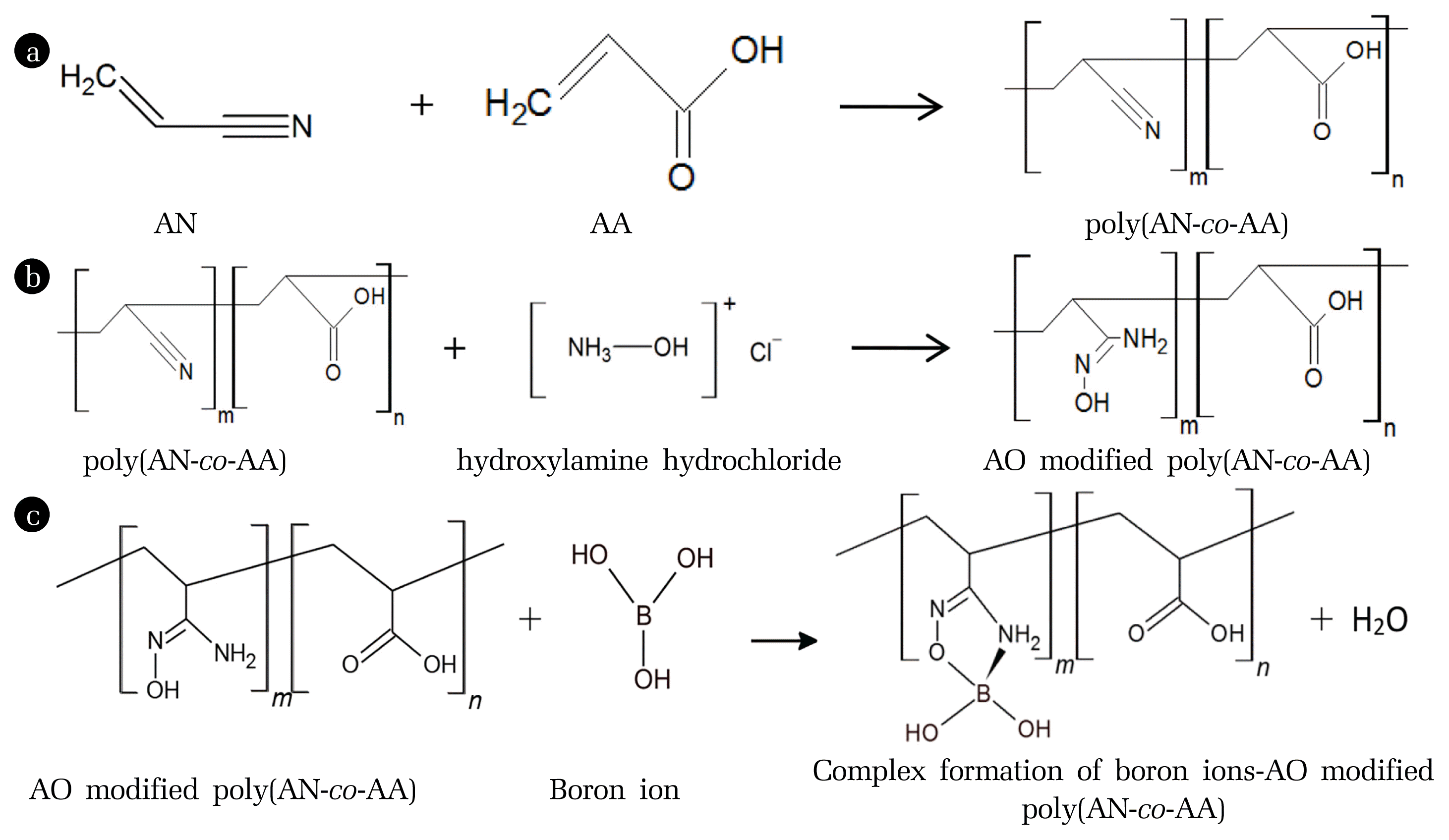



Application Of Feed Forward And Recurrent Neural Network In Modelling The Adsorption Of Boron By Amidoxime Modified Poly Acrylonitrile Co Acrylic Acid



C March 21 Browse Articles



Boron The Periodic Table At Knowledgedoor
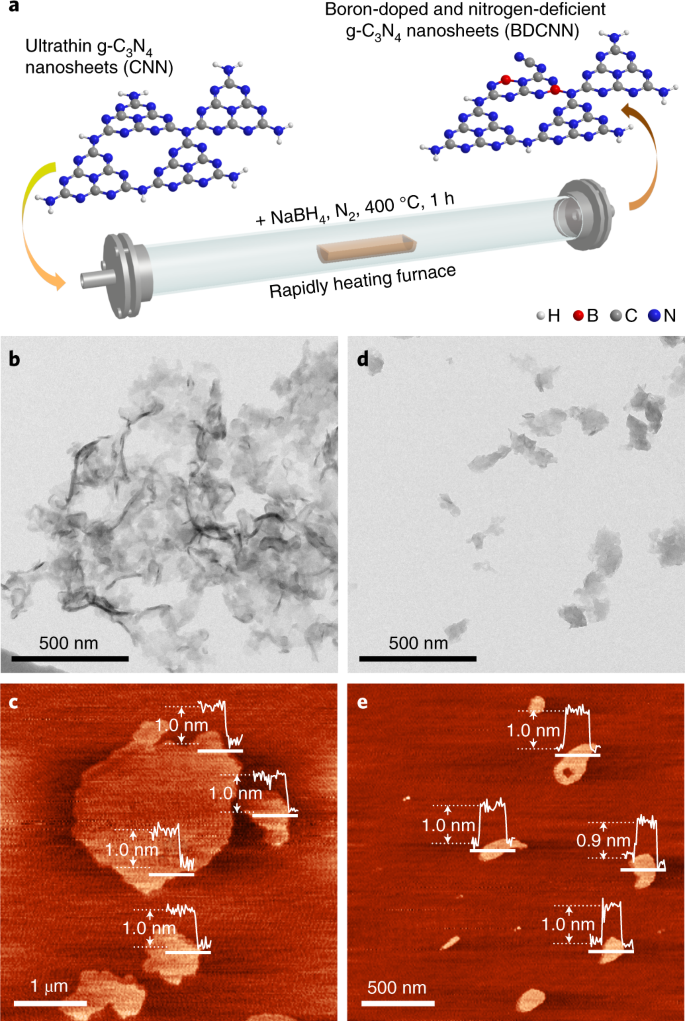



Boron Doped Nitrogen Deficient Carbon Nitride Based Z Scheme Heterostructures For Photocatalytic Overall Water Splitting Nature Energy



Bg Seawater Ph Reconstruction Using Boron Isotopes In Multiple Planktonic Foraminifera Species With Different Depth Habitats And Their Potential To Constrain Ph And Pco2 Gradients




Nucleophilic Neutral Diborane 4 Compounds With Sp3 Sp3 Hybridized Boron Atoms Himmel 18 European Journal Of Inorganic Chemistry Wiley Online Library




Atomic Arrangement And Mechanical Properties Of Chemical Vapor Deposited Amorphous Boron Sciencedirect
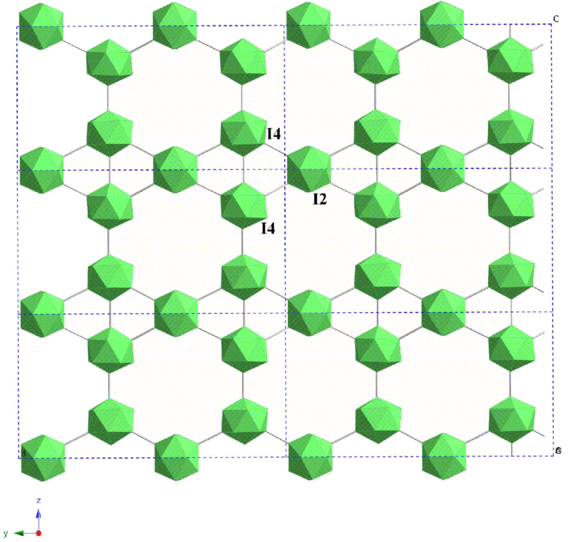



Crystal Structure Of Boron Rich Metal Borides Wikiwand




Webelements Periodic Table Boron Properties Of Free Atoms




The Boron Content Of Selected Foods Comparison Of Food Composition Download Table




What Is Boron Where Is Boron Used




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom Youtube
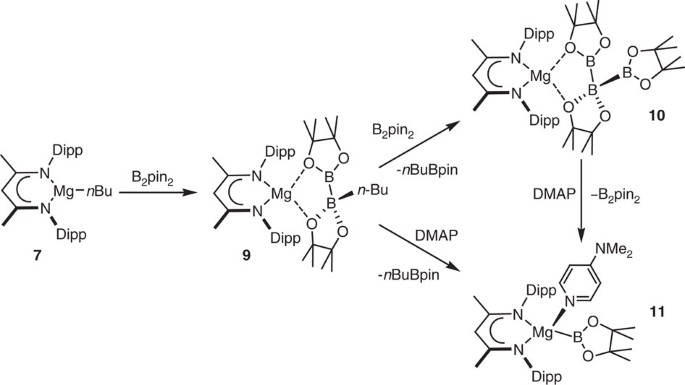



Easy Access To Nucleophilic Boron Through Diborane To Magnesium Boryl Metathesis Nature Communications
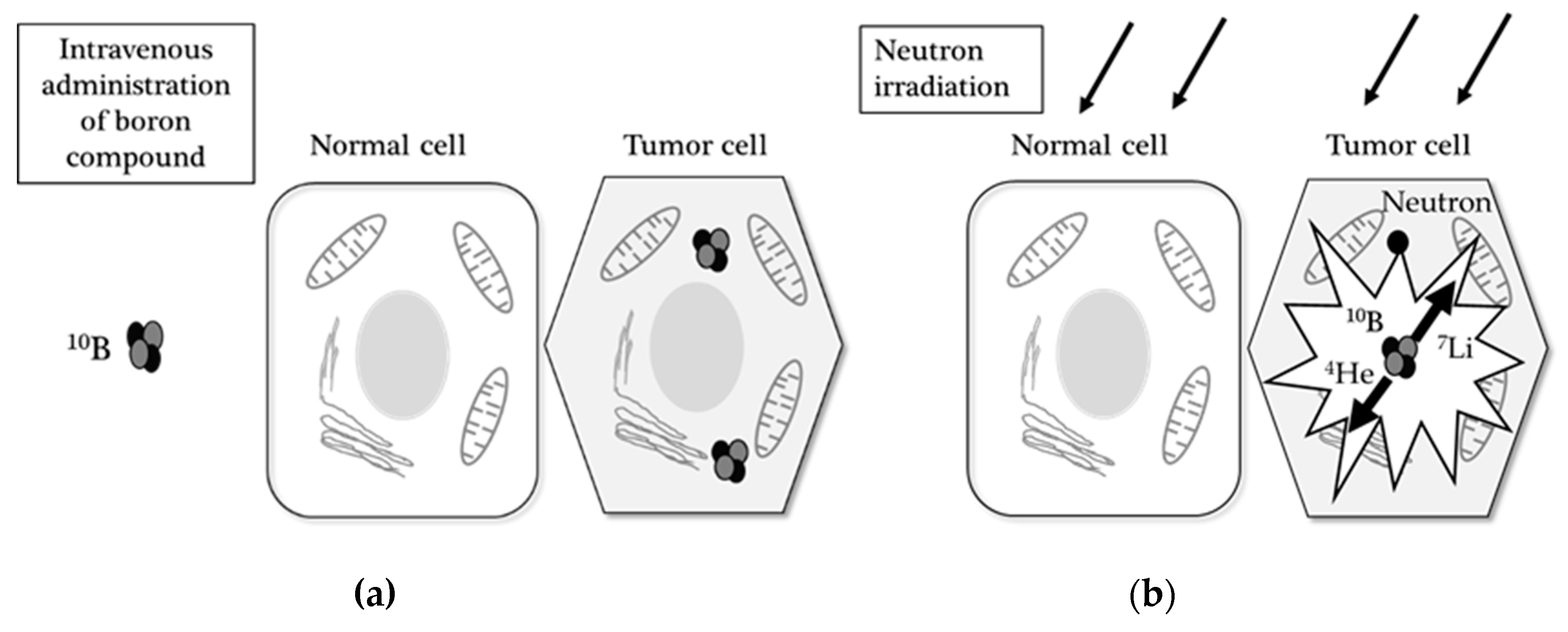



Cancers Free Full Text Boron Neutron Capture Therapy And Photodynamic Therapy For High Grade Meningiomas Html




Materials Free Full Text Influence Of Amorphous Boron Grain Size High Isostatic Pressure Annealing Temperature And Filling Density Of Unreacted Material On Structure Critical Parameters N Value And Engineering Critical Current Density



Tel Archives Ouvertes Fr Tel Document




Simultaneous Removal And Recovery Of Boron From Waste Water By Multi Step Bipolar Membrane Electrodialysis Sciencedirect




Application Of Feed Forward And Recurrent Neural Network In Modelling The Adsorption Of Boron By Amidoxime Modified Poly Acrylonitrile Co Acrylic Acid




Materials Free Full Text Influence Of Amorphous Boron Grain Size High Isostatic Pressure Annealing Temperature And Filling Density Of Unreacted Material On Structure Critical Parameters N Value And Engineering Critical Current Density




The Relative Cohesive Energy Ev Values Per Atom For Boron Clusters N Download Table




Nanomaterials Free Full Text Creation Of Negatively Charged Boron Vacancies In Hexagonal Boron Nitride Crystal By Electron Irradiation And Mechanism Of Inhomogeneous Broadening Of Boron Vacancy Related Spin Resonance Lines Html



3



Www Diva Portal Org Smash Get Diva2 Fulltext01 Pdf




Binding Energies In Ev For The Boron Target States Included In Our Cc Download Scientific Diagram




Periodic Trends Periodic Properties Ppt Download
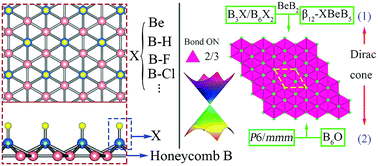



Realizing Graphene Like Dirac Cones In Triangular Boron Sheets By Chemical Functionalization Journal Of Materials Chemistry C Rsc Publishing




Boron Derivative An Overview Sciencedirect Topics




Materials Free Full Text Influence Of Amorphous Boron Grain Size High Isostatic Pressure Annealing Temperature And Filling Density Of Unreacted Material On Structure Critical Parameters N Value And Engineering Critical Current Density




Exploring Boron Applications In Modern Agriculture A Structure Activity Relationship Study Of A Novel Series Of Multi Substitution Benzoxaboroles For Identification Of Potential Fungicides Sciencedirect




Bg Seawater Ph Reconstruction Using Boron Isotopes In Multiple Planktonic Foraminifera Species With Different Depth Habitats And Their Potential To Constrain Ph And Pco2 Gradients




Scielo Brasil The Effect Of Adding Boron In Solidification Microstructure Of Dilute Iron Carbon Alloy As Assessed By Phase Field Modeling The Effect Of Adding Boron In Solidification Microstructure Of Dilute Iron Carbon
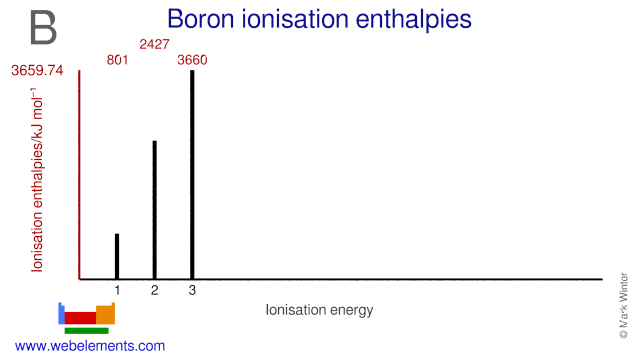



Webelements Periodic Table Boron Properties Of Free Atoms



Www Osti Gov Etdeweb Servlets Purl



Aip Scitation Org Doi Pdf 10 1063 5




Calculated Two Dimensional And Experimental Values For Hexagonal Download Table




Atomic Arrangement And Mechanical Properties Of Chemical Vapor Deposited Amorphous Boron Sciencedirect




Stabilization Of Neutral Tricoordinate Pyramidal Boron Enhanced Lewis Acidity And Profound Reactivity Sciencedirect




Measured Values Of D 11 B For Different Chemical Extractions Of Boron Download Table




Boron And Borates Market Research In The Cis Infomine Research



1




1 Heating Values Of Various Fuels 1 2 Download Table




Nanomaterials Free Full Text Creation Of Negatively Charged Boron Vacancies In Hexagonal Boron Nitride Crystal By Electron Irradiation And Mechanism Of Inhomogeneous Broadening Of Boron Vacancy Related Spin Resonance Lines Html
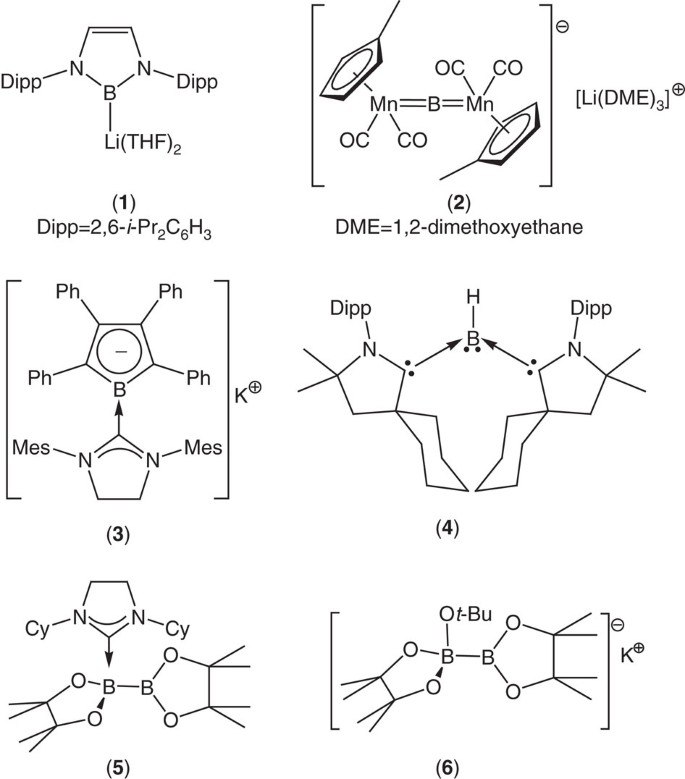



Easy Access To Nucleophilic Boron Through Diborane To Magnesium Boryl Metathesis Nature Communications




Effects Of Boron Containing Compounds In The Fungal Kingdom Sciencedirect




Materials Free Full Text Influence Of Amorphous Boron Grain Size High Isostatic Pressure Annealing Temperature And Filling Density Of Unreacted Material On Structure Critical Parameters N Value And Engineering Critical Current Density
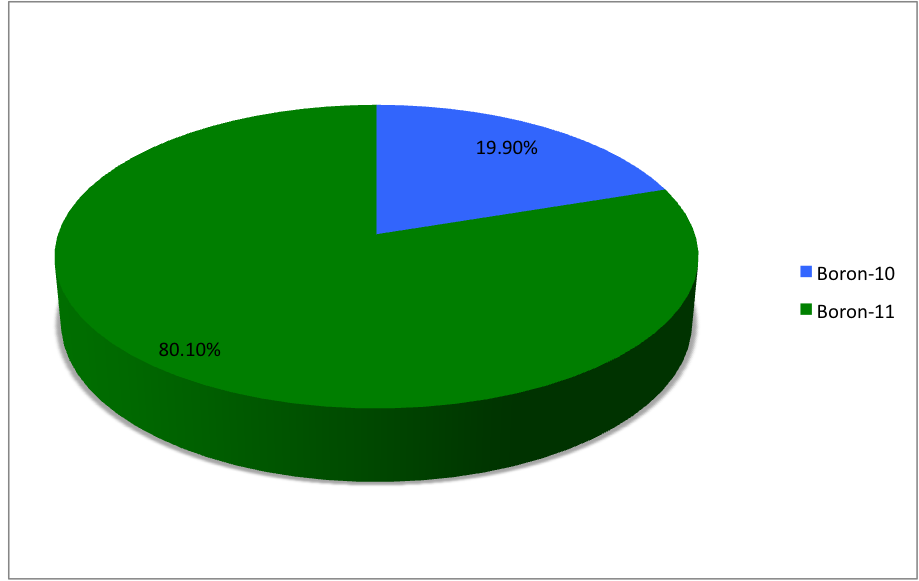



Isotopes Of Boron Wikipedia



Hal Archives Ouvertes Fr Hal 0446 Document




The I1 Values Of Li Be And Care 5 4e Atom 9 3ev Atom The I1 Value At Boron Is Brainly In




Boron Derivative An Overview Sciencedirect Topics




Boron Isotopic Analyses Of Nbs Srhj 951 Standard Download Table



1




Draw A Lewis Structure Of Borane Bh3 Note That The Boron Atom Does Not Have An Octet A Which Is More Electronegative B Or H Circle One B Which Atom Of



2
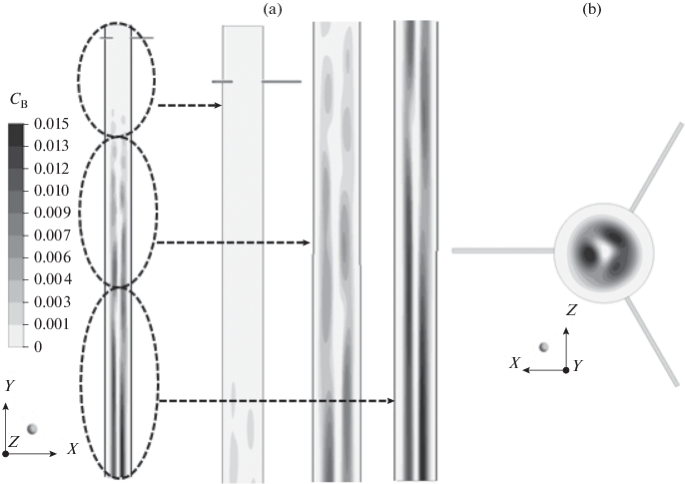



Comparative Study Of Gas Dynamic Processes In Inductively Coupled Argon Hydrogen Plasma Containing Boron Trichloride And Boron Trifluoride Springerlink




Scielo Brasil The Effect Of Adding Boron In Solidification Microstructure Of Dilute Iron Carbon Alloy As Assessed By Phase Field Modeling The Effect Of Adding Boron In Solidification Microstructure Of Dilute Iron Carbon




Room Temperature Coherent Control Of Spin Defects In Hexagonal Boron Nitride Science Advances




Boron And Borates Market Research In The Cis Infomine Research




Nanocrystalline Diamond Films Heavily Doped By Boron Structure Optical And Electrical Properties




Boron 10 B Pubchem



Boron Nmr




Bg Seawater Ph Reconstruction Using Boron Isotopes In Multiple Planktonic Foraminifera Species With Different Depth Habitats And Their Potential To Constrain Ph And Pco2 Gradients




Photoelectron Spectra And Electronic Structure Of Boron Diacetate Formazanates Sciencedirect



Oatao Univ Toulouse Fr 1 Korycki Pdf
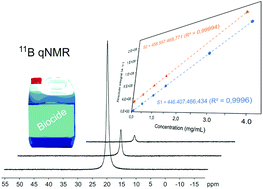



Pushing The Frontiers Boron 11 Nmr As A Method For Quantitative Boron Analysis And Its Application To Determine Boric Acid In Commercial Biocides Analyst Rsc Publishing




Boron Containing Radical Species Sciencedirect



Tel Archives Ouvertes Fr Tel Document




Materials Free Full Text Influence Of Amorphous Boron Grain Size High Isostatic Pressure Annealing Temperature And Filling Density Of Unreacted Material On Structure Critical Parameters N Value And Engineering Critical Current Density




Crystal Structure Of Boron Rich Metal Borides Wikiwand




A New Era For Boron Nitride From Medicine To Quantum Information




Boron And Borates Market Research In The Cis Infomine Research



Nano Bio Ehu Es Files Paulgiraud Masterthesis Pdf




Pdf Novel Bi Nuclear Boron Complex With Pyrene Ligand Red Light Emitting As Well As Electron Transporting Material In Organic Light Emitting Diodes Semantic Scholar




Application Of Feed Forward And Recurrent Neural Network In Modelling The Adsorption Of Boron By Amidoxime Modified Poly Acrylonitrile Co Acrylic Acid
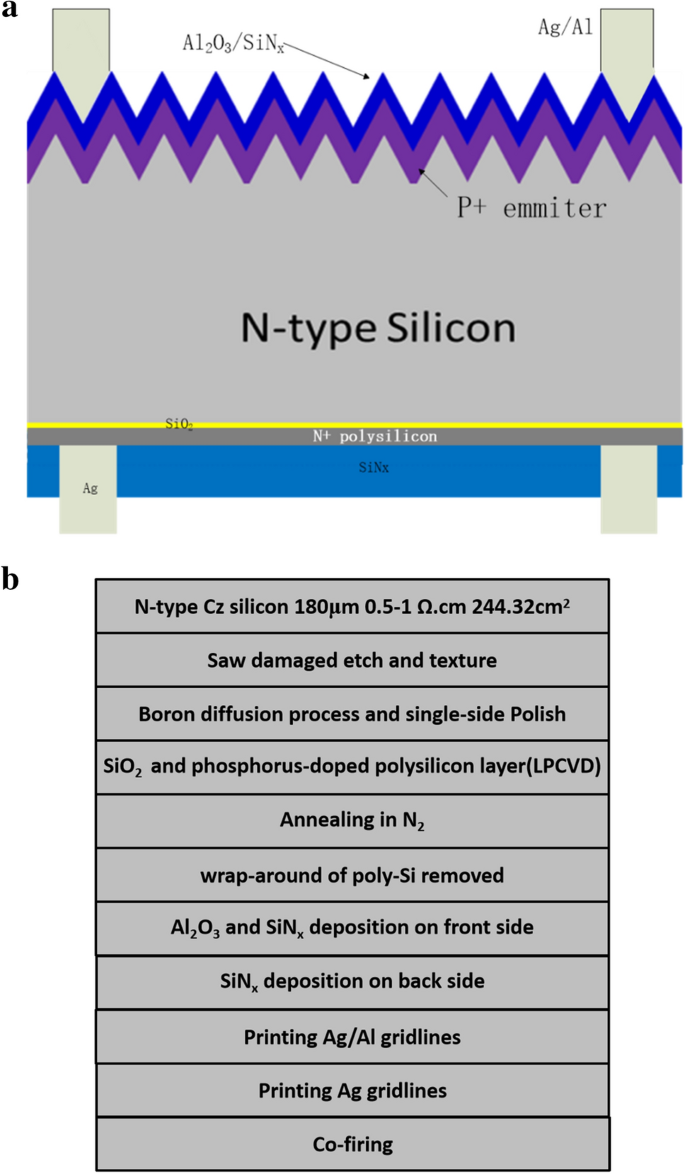



Study Of Boron Diffusion For P Emitter Of Large Area N Type Topcon Silicon Solar Cells Springerlink




Investigations Over Optical Properties Of Boron Complexes Of Benzothiazolines Sciencedirect




Pdf Effect Of Deficit And Over Standard Boron Content In Nutrient Solution On The Biological Value Of Tomato Fruit
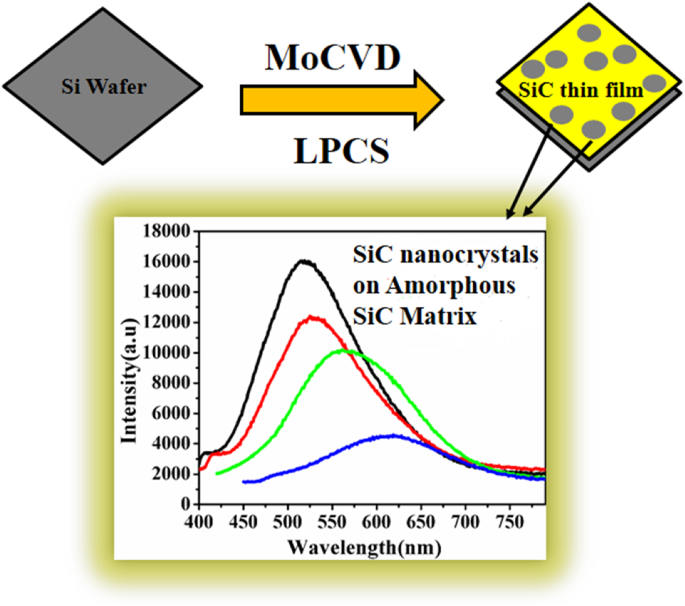



Enhancement Of Optical Properties Of Boron Doped Sic Thin Film A Sic Qd Effect Springerlink




Pdf Electron Affinities Of Boron Aluminum Gallium Indium And Thallium Pekka Pyykkoe Ephraim Eliav And Pekka Pyykko Academia Edu



Boron The Periodic Table At Knowledgedoor




Scielo Brasil The Effect Of Adding Boron In Solidification Microstructure Of Dilute Iron Carbon Alloy As Assessed By Phase Field Modeling The Effect Of Adding Boron In Solidification Microstructure Of Dilute Iron Carbon



0 件のコメント:
コメントを投稿