17 The I1 values of Li, Be and C are 54 eV/atom, 932 eV/atom and 1126 eV/atom The I1 value of Boron is 1) 136 eV/atom 2) 9 eV/atom 3) 145 eV/atom 4) 215 eV/atom 18 The I1 of potassium is 4339 eV/atom, the I1 of sodium 1) 4339 2) 221 3) 5138 4) 1002 wwwsakshieducationcom wwwsakshieducationcom a) The first ionization energies (I1) of these elements are lower than the corresponding values for Group 2 elements However, the second (I2) and third (I3) ionization energies are much higher This is because the first electronThe heats of formation at 25° C, from amorphous boron and hydrogen, are 673 ± O52 kcal/ mole for diboranc (gas) >l nd 1299 ± O39 for pentaborane (gas) 1 Introduction In order Lo ascertain the bond energy relations in the boron hydrides, precise values of the heats of forma bon of these substances from the elements are necded
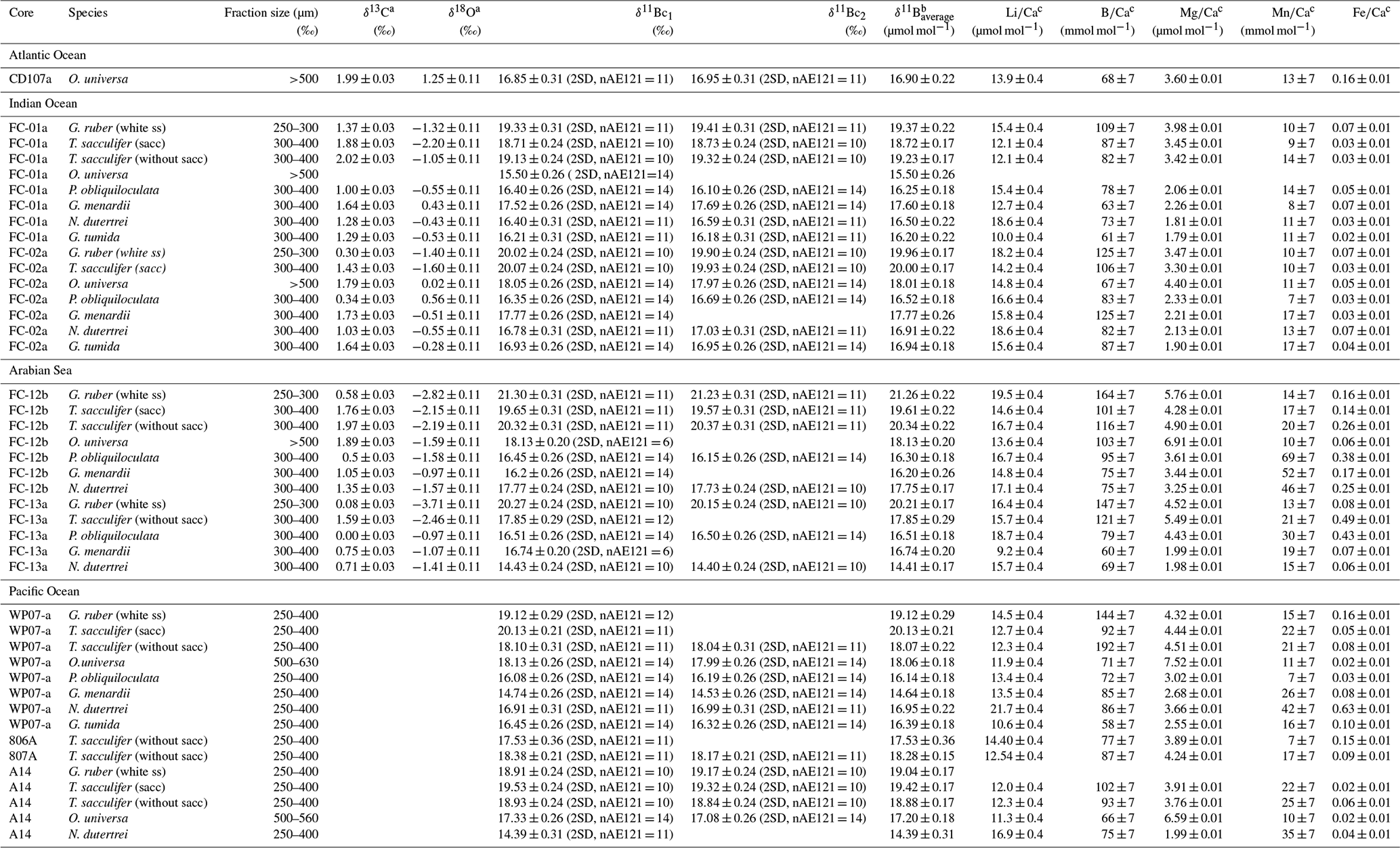
Bg Seawater Ph Reconstruction Using Boron Isotopes In Multiple Planktonic Foraminifera Species With Different Depth Habitats And Their Potential To Constrain Ph And Pco2 Gradients
Is boron valuable





